
Trial Data DP
临研数据一体化平台
Trial Data APP 临床试验移动管家
一款操作灵活,便利的临床试验移动管家
Trial Data APP拍照上传病历 (数据源) 图片至Trial Data EDC,拥有去隐私化功能,将受试者信息去隐私化后上传,有效保障受试者隐私信息安全。同时移动端APP可以支持在线随机,让研究者在没有电脑的情况下也可便利进行随机操作。Trial Data APP还支持移动端数据录入和临床数据移动端查阅功能。
 应用宝下载
应用宝下载
 apk下载
apk下载
-
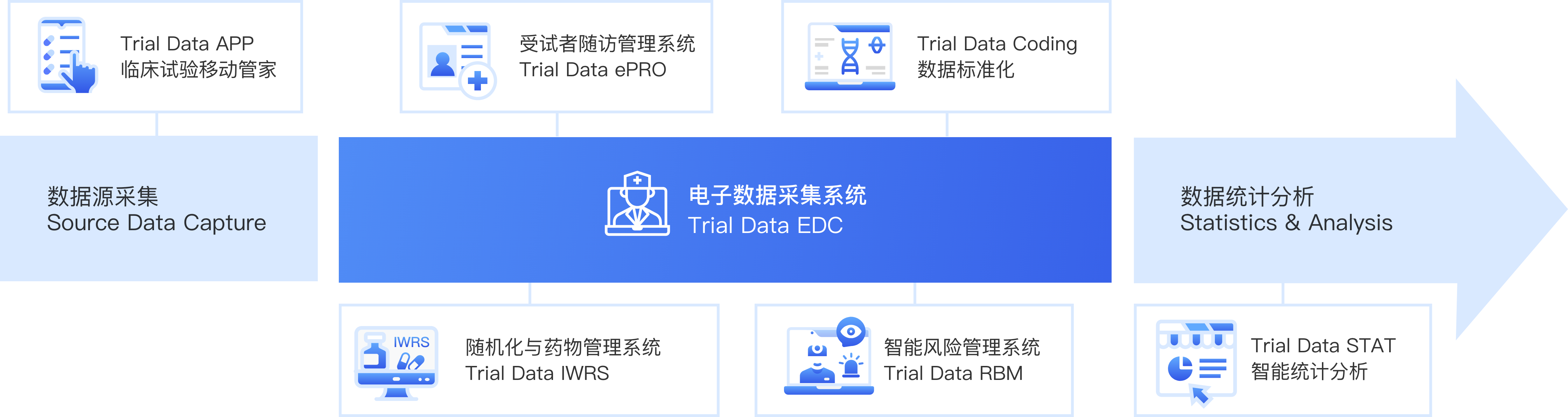
数据源采集
可以保证数据真实可靠,随时可以查看。CRC、CRA不需要等医生下班才能查看数据源,系统提供高效便利地工作。 -
随机工作
医生不再受时间和空间的限制,(即使是手术室或是门诊)只需要打开手机APP就可以完成随机工作。 -
快速录入
不需要等待科室电脑空闲才进行录入随时都可以快速录入。
Trial Data IWRS 随机化与药物管理系统
以受试者为中心,支持多中心临床试验的药物随机和管理系统Trial Data IWRS可以有效管理多中心临床试验的随机发药,解决跨地域各分中心进度不同,药物过期或过剩等问题。不同地理位置的研究者可以随时进行受试者入组登记、筛选、随机和药物发放和紧急破盲操作。多种随机方案最大化的保证临床研究的科学性。友好的提醒机制,让临床研究的药物管理更快、更优、更精确。同时IWRS和受试者端以及EDC已进行无缝衔接,避免数据不一致以及重复录入等操作问题。

-
多系统无缝衔接
支持移动端和电脑端双向操作,高效便捷。多系统无缝对接,满足一站式临床研究需求。 -
盲态配发
满足盲态项目对药物的管理与配发需求,可实现药物的动态管理,降低药品损耗。 -
智能库存管理
智能库存、临期提醒,保障项目高效运营。 -
线上揭盲
线上药物揭盲管理,安全可靠。
Trial Data EDC 电子数据采集系统
一款遵循法规指南的EDC系统,并基于临床试验中常见问题开发了人性化的辅助功能,帮助提高科研效率,节省研发成本。
-
高效
模块化快速构建eCRF,丰富的模板库支持CDASH建库核查规则支持多字段,多访视快速适配项目正式上线前系统自动校验 -
智能
支持核查规则通配及列表内外交叉校验(无需编程)支持跨访视,跨页面核查支持多字段复合逻辑比较支持逻辑核查功能复制质疑提醒方式多样化 -
便捷
数据源去隐私化处理,支持远程录入手机端移动录入OCR智能录入移动端随机数据一次录入,全平台共享 -
专业
21 CFR part 11国际标准GAMP5系统验证标准CDISC专业会员IS027001信息安全管理认证WHO-UMC国际认证供应商国家三级等保认证
Trial Data ePRO 受试者随访管理系统
智能随访管理,链接医生与受试者的随访桥梁通过Easy Fu关联受试者微信端,及时收到随访提醒,有效提高随访的依从性并减少受试者脱落率,自动推送问卷量表,受试者在线填写提交。同时支持受试者在研究过程中随时记录不良事件、合并用药、受试者病情日志等信息,有效降低随访期间关键信息缺失,医患随时沟通,研究者或CRC可与多个受试者同时进行线上一对多沟通和答疑,自动生成电话随访计划 并支持电话过程中录音和快速在线记录受试者情况,提高电话随访效率。
-
随访提醒
避免人为原因的遗漏 从而降低受试者失访率 -
自动推送问卷量表
受试者在线填写,支持受试者填写不适记录等,降低随访关键信息缺失 -
医患微信互动
受试者感知到医生的关心 提高受试者依从性 -
电话录音
提供真实、可靠、高质量的数据源随时进行质控核查
Trial Data ERM 智能风险管理系统
智能化多维度报告辅助项目管理,将项目风险量化可控,实时监测。
Trial Data ERM可确保您遵循ICH-GCP E6 (R2) GCP要求,在项目中实施远程风险监控。
Trial Data 基于法规要求和行业国际标准,通过统计学算法将项目风险量化呈现,方便项目组实时监测并进行有效干预。
Trial Data 基于法规要求和行业国际标准,通过统计学算法将项目风险量化呈现,方便项目组实时监测并进行有效干预。
-
风险监控
分析数万的数据点,标识项目风险
风险等级自动输出,针对性制定对策
协助项目管理层实时了解项目关键风险 -
动态监查
项目监查可基于风险等级动态调整
按风险等级及优先级自动生成监查计划
便于提高CRA效率,助力提高项目质量 -
绩效管理
可根据角色进行绩效考核
查询CRA/PM的绩效表现
可筛选site了解项目运营中的既往情况
Trial Data Coding 数据标准化
通过WHODrug系统认证,让医学编码变得更快、更经济支持 MedDRA、WHODrug 等医药相关词典、多种搜索方式、批量编码和批量审核、同义词库和质疑词库的维护、项目内编码,支持官方词典一键上传,按项目分配权限,自动编码与手工编码相结合。信息化系统解决了手工编码过程繁琐,编码前后不一致的问题,也避免手工编码的可能错误,有效提升编码工作的效率与质量。
-
支持不同治疗领域,不同的同义词词典
-
支持不同项目/申办方设置指定编码路径字典
-
支持多个项目同样的逐字术语汇总编码
-
支持账号权限按照项目管理
-
支持与您的所有源系统自动同步

Trial Data STAT 智能统计分析
智能在线统计专家,您身边的统计专家我们通过R语言开发的智能在线统计专家,无需数据导出,无需统计学专业知识,即可实时在线完成统计分析,生成专业的统计图表并支持在线图表编辑功能。包含一般描述,单因素分析,比较,多因素分析,生存分析等。




















