- 创达远程智能临床研究全流程服务 -
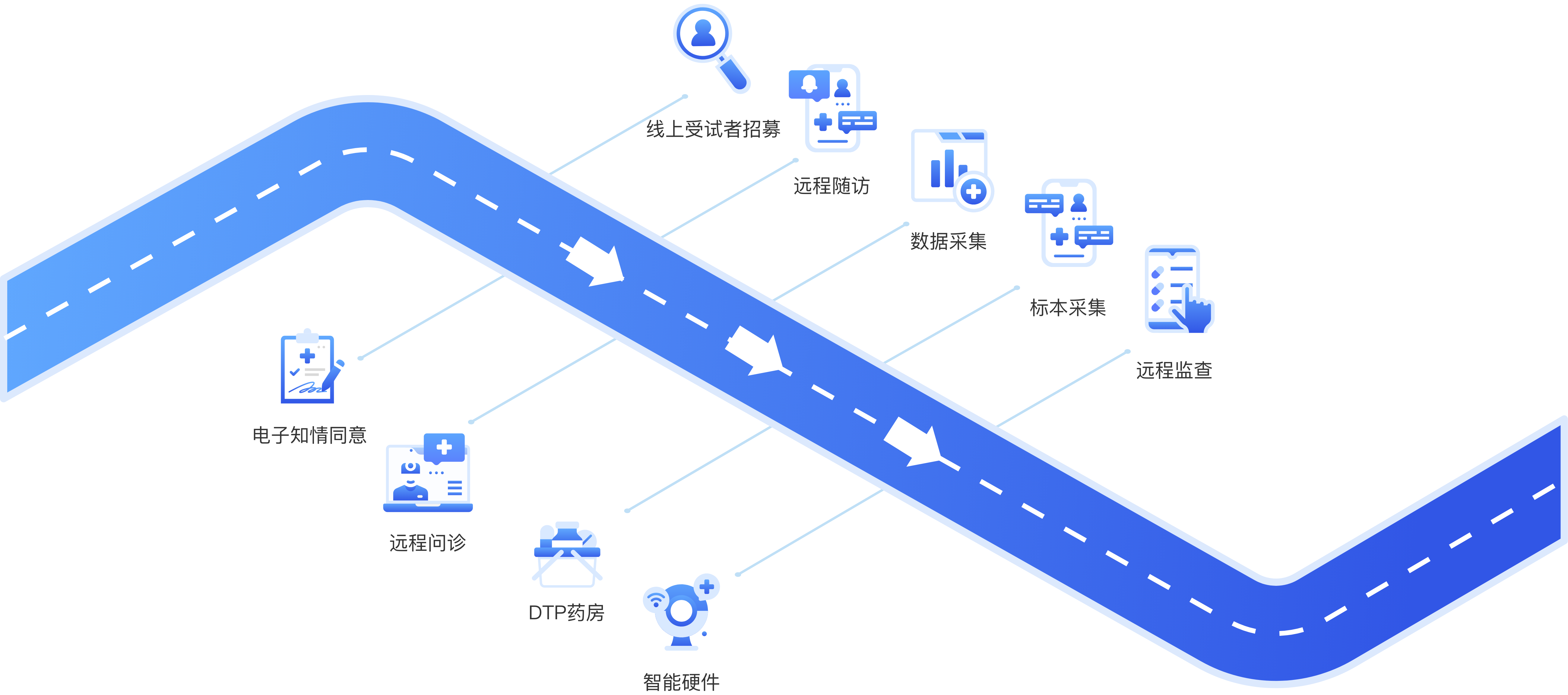
- 创达丰富的产品线,为临研全面赋能 -
Trial Data OS
远程智能临研操作平台Trial Data DP
临研数据一体化平台Trial Data OA
GCP机构一体化管理平台- 我们提供的整体解决方案 -
- 专业认证与资质 -
系统符合行业标准
21 CFR part 11(国际基础标准)
GAMP5(系统验证标准)
CDISC (数据互交标准)
ISO27001 信息安全管理认证
国家临床试验的电子数据采集技术指导原则
GAMP5(系统验证标准)
CDISC (数据互交标准)
ISO27001 信息安全管理认证
国家临床试验的电子数据采集技术指导原则
国际标准
General Principles of Software Validation
Final Guidance for Industry and FDA Staff
Guidance for Industry - COMPUTERIZED
SYSTEMS USED IN CLINICAL TRIALS
Final Guidance for Industry and FDA Staff
Guidance for Industry - COMPUTERIZED
SYSTEMS USED IN CLINICAL TRIALS





- 我们的成就 -
-
50+
企业客户
-
200+
项目数
-
1,000+
医院合作
-
60,000+
服务受试者数















